Using a dilution refrigerator is the most powerful way to reach very low temperatures (a few thousands of degree above the absolute zero). It is an essential tool of our lab since many interesting effects in mesoscopic physics take place at these temperatures. In the Quantum Transport group we have 4 dilution fridges available. Each of them has slightly different characteristics, but the underlying principle is the same.
Any refrigerator needs some thermal isolation from the ambient temperature and a cooling fluid. In a dilution fridge, good thermal isolation is achieved by surrounding the coldest part of the fridge by many metallic shields held at low temperatures, to prevent heating by radiation. Between these shields, an isolation vacuum prevents heating by thermal conduction. The cooling fluid is a mixture of the two isotopes of Helium Both 4He and 3He boil (under ambient pressure) at very low temperatures (4.2K for 4He and 3.2K for 3He), which make them suitable to reach temperatures of about 1K, but the mixture of these two isotopes has even more interesting thermodynamical properties allowing to obtain lower temperatures.
The principle of operation of the dilution refrigerator was originally proposed by H. London in 1951. When a mixture of the two isotopes of helium is cooled below a critical temperature, it separates into two phases. The higher "concentrated phase" is rich in 3He and the heavier "dilute phase" is rich in 4He. Since the enthalpies of the 3He in the two phases are different, it is possible to obtain cooling by "evaporating" the 3He from the concentrated phase into the dilute phase. This process continues to work even at the lowest temperatures because the equilibrium concentration of 3He in the dilute phase is still finite even as the temperature approaches absolute zero.

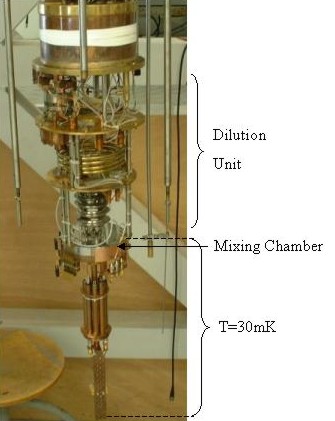
The picture above is a photograph of the inside of an Oxford 200S dilution fridge. During the operation of the fridge, the mixture continuously circulates through the complex heat exchangers, flow impedances, ... which constitute the Dilution Unit. It is put into motion by an external pump. The phase separation takes place at the lowest part which is called the Mixing Chamber. This is the very heart of the fridge, its coldest part. The sample which is to be cooled is thermally anchored to the Mixing Chamber by a copper piece.